Uganda’s Ebola-free status leaves vaccine trial bid in disarray
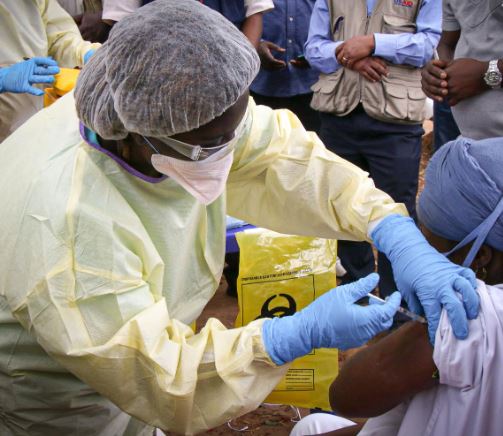
The World Health Organization and some scientists disagreed on the modalities of trying the Ebola vaccine in Uganda after the country was declared Ebola-free.
The three vaccine-producing companies now have no clear path to testing the Ebola vaccine that was meant to combat Ebola in Uganda.
According to Health Policy Watch, three candidate vaccines – SUDV, Sabin (ChAD3), and Oxford (ChAdOx1)—were presented to Uganda in mid-December with roaring applause.
But on January 11, 2023, Uganda declared the end of a four-month Ebola outbreak that had been difficult to contain but was eventually quickly under control.
The WHO and other scientists who participated in a conference could not agree on how to test the vaccines now that Uganda has been declared Ebola-free.
The scientists also discussed options including regulatory approval based on animal-only studies.
The other option is through immunobridging, which the US Food and Drug Administration describes as a “regulatory and scientific approach to infer vaccine effectiveness through comparison of immune response markers elicited by a vaccine under different sets of conditions.”
This technique was also employed in the production of COVID-19 vaccines.
It can also be used to alter dosage and formulation. In some cases, animal trial findings have been extrapolated to humans, including the approval of Ebola vaccines and treatments.
During the meeting last week on Thursday, Ana Maria Hanao-Restrepo of the WHO asked participants if “anything could be done with the thousands of doses of the three candidate vaccines that are currently in Uganda to prepare for the next outbreak.”
According to Health Policy Watch, some responses included suggestions for more nonhuman primate efficacy data, testing the effects of one vaccine dose versus two; which vaccine provided rapid protection versus which provided long-term protection, and whether efficacy was affected by malaria.
While efficacy studies in mice and guinea pigs were “not very applicable,” non-human primate studies, particularly in cynomolgus macaques, were similar to human progression and had been extensively used for Ebolavirus Zaire studies, according to CEPI’s Bill Dowling.
However, Dowling stated that there were no assays in place to support trials, including Sudan virus materials.
However, Dowling stated that there were no assays in place to support trials, including Sudan virus materials.
This would include an expedited approval pathway in which efficacy is based on a surrogate endpoint that is likely to predict clinical benefit, conditional approval in which the benefit of the vaccine’s immediate availability to patients is deemed greater than the risk, and an exceptional circumstances pathway in which the sponsor is not able to provide specified data.
“The US animal rule is very stringent, and does not apply if approval can be based on an efficacy standard elsewhere in FDA regulations.”
“The animal rule in the United States is very stringent, and it does not apply if approval can be based on an efficacy standard elsewhere in FDA regulations,” said Dowling,
And that it was usually accompanied by “a large number of studies with a large number of animals and was quite a lengthy process.”
Nancy Sullivan, director of Boston University’s National Emerging Infectious Diseases Laboratories, believes that efficacy data must be generated in humans.
“We must be cautious when attempting to compare vaccines based on immunogenicity. I think we need those efficacy results, and if it takes multiple trials, we should do it,” Sullivan stressed.
Uganda was declared Ebola-free after months of containment measures to curb cases. Kampala recorded over 100 cases over the months of the outbreak.
Ebola vaccines due for trial
-SUDV
– Sabin (ChAD3)
-Oxford (ChAdOx1)


